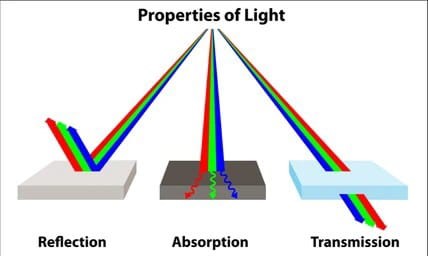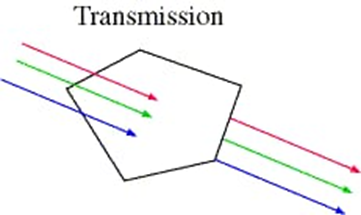
The transmission of Light is defined as the moving of the electromagnetic waves through a material.
Visible light is the cause we are able to see anything at all. Transmission of light moves as sea waves, bouncing off objects so we can see them. Without it, we’d be unable to see in darkness. But, in physics, light can refer to any kind of electromagnetic wave i.e; radio waves, microwaves, infrared, visible light, ultraviolet, X-rays, or gamma rays.
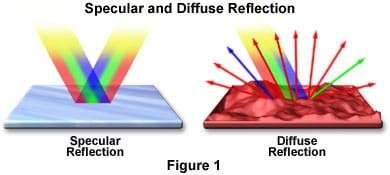
When we shine light on an object, a number of the things can happen. Reflection of light happens when light bounces off of a surface. Specular reflection is when the light reflects off of a shiny surface like a mirror. Diffuse reflection is however when light illuminates a dull object.
Transmission of light happens when light waves move all the way through a piece of material without being absorbed.
Transmittance
When the light waves moves through a transparent (or semi-transparent) material, it can be either transmitted, absorbed, or reflected. The transmittance of a material is the proportion of the incident light that moves all the way through the other side.
For an example, let’s us say that we’re shining a flashlight on a semi-transparent glass block. We start off with 100% of our incident light. The first thing that happens is that 30% of that light is reflected off the outer surface of the glass. This leaves it with 70% to continue through the glass block. Another 50% of the light is absorbed by the molecules inside the glass block itself. That leaves the material with 20% that emerges from the opposite side. So, we could say that the glass block has a transmittance of 20%.
The transmittance of the material depends on its thickness, but it also depends on the type of the light (or electromagnetic waves) that we are using.
Absorption
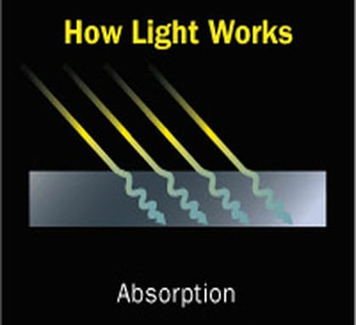
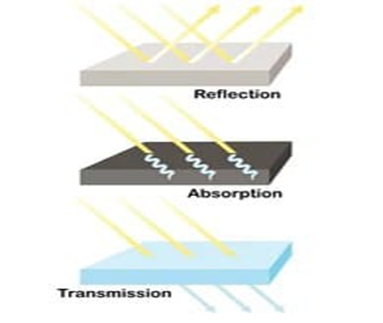
Light absorption is the process by which the light is being absorbed and converted into energy. Absorption of light depends on the electromagnetic frequency of the light and object’s nature of atoms. Thus, we can say that the absorption of light is directly proportional to the frequency. If they are complementary, light is being absorbed.
The absorption of light depends on the state of an object’s electron. All electrons vibrate at a specific frequency, which is known as the “natural” frequency. When light interacts with an atom of the same frequency, the electrons of the atom becomes excited and start vibrating. During this vibration, the electrons of the atoms interact with the neighboring atoms and this vibrational energy convert into the thermal energy. Consequently, the light energy is not to be seen again, that is why absorption differs from reflection and transmission and since different atoms and molecules have different natural frequencies of vibration and thus energy is generated, they selectively transmits different visible lights of different frequencies.
For example, grass appears green in white light: red, orange, yellow, blue, indigo and violet (such colors are absorbed) by the grass. Green light is reflected by the grass and detected by our eyes.
In the absorption, the frequency of an incoming light wave is at or near the energy levels of the electrons in the matter. The electrons absorbs the energy of the light wave and changes their energy state.
The absorbance of an object results how much of the incident light is being absorbed by it (instead of being thus reflected or refracted). This may be thus related to the other properties of the object through the Beer–Lambert law.
Relationship between Absorption and Transmission
The absorbance has the logarithmic relationship to the transmittance; with an absorbance of 0 corresponding to a transmittance of 100% and an absorbance of 1 corresponding to 10% transmittance.
Transmission of the light is the ratio of the radiant power being transmitted by an object to the radiant power incident on the same object. Absorption of light is the negative logarithm of transmittance.
Transmittance (T) is the fraction of the incident light which is being transmitted. In other words, we can say it’s the amount of light that “successfully” passes through the substance and comes out through the other side. The absorbance (A) of the light is the flip-side of transmittance and states how much of the light the object absorbed.
Difference between Transmission and Absorption
The main difference between the absorbance of the light and transmittance of light is that the absorbance measures how much of the incident light is being absorbed when it travels in through a material while transmittance measures how much of the light is being transmitted.