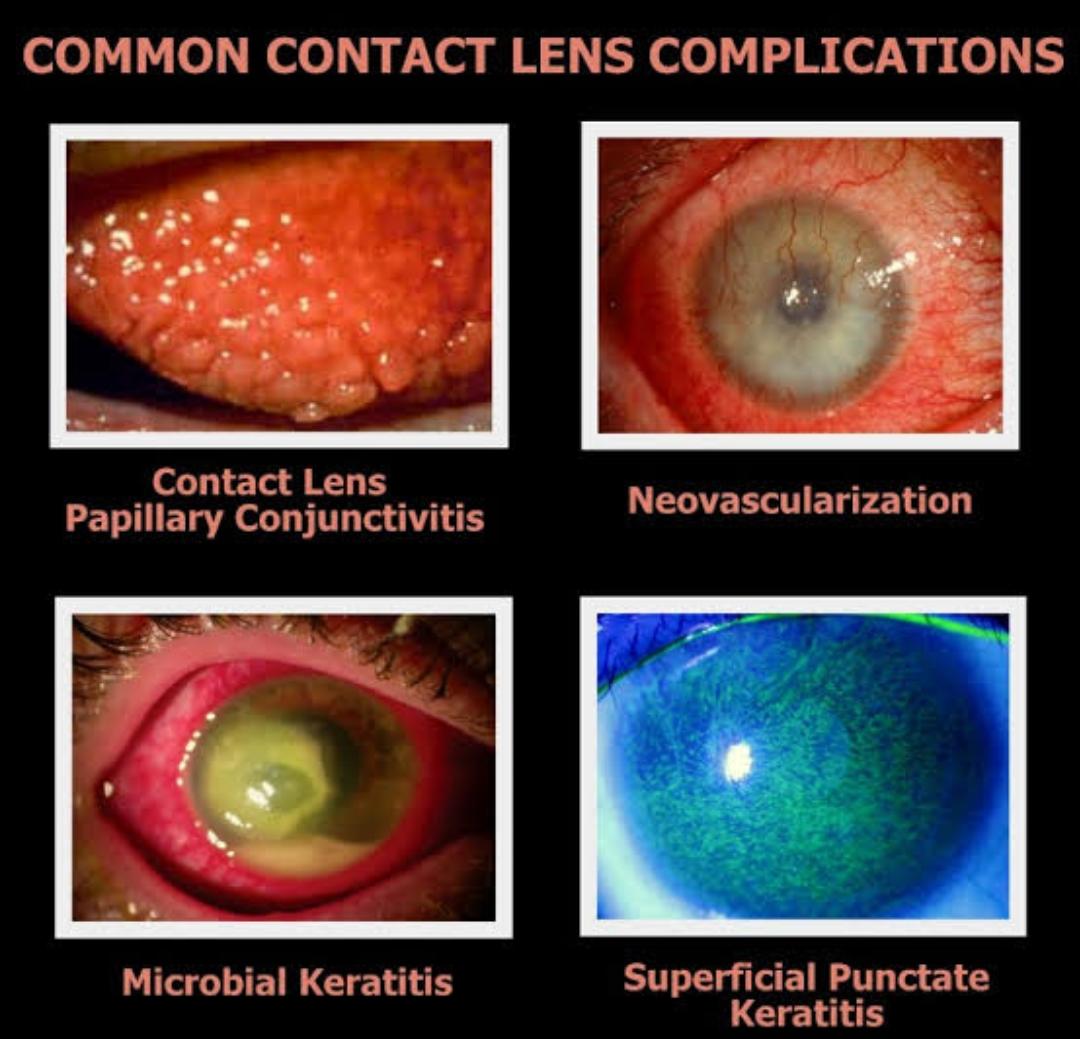
When the lens is received from the manufacturer it is sterile, so
where does the infection come from? Clearly, most of the
complications with contact lens are because of the poor
compliance and improper usage of the lens care systems. It is
the responsibility of the practitioner to understand first and then
explain the proper usage of each care system. Though the specific components of these systems vary with the type of the lens either soft or hard, mostly include a lens cleaner, rinsing solution, disinfecting and storing solution. Now a days, multipurpose solutions which perform all these functions, are popular owing to their ease of use and convenience.
When a patient is prescribed contact lenses, he/she must be given comprehensive and easy to follow instructions about the care of lenses because apart from monetary loss, damage to the lens may result in poor optics and can cause irreversible damage to the eyes.
Purpose and Objective of lens care system
- To remove deposits and microorganisms
- To enhance lens comfort
- To reduce the risk of infection and irritation
- To provide best vision
- To maintain adequate lens wetting and stabilize all the lens parameters
A rationale for disinfecting contact lenses
The eye has a number of inherent protective mechanisms to prevent infections. Tear film and blinking play a vital role in the resistance of infection. Tear production of the order of 1-2 micro liter/min and overall tear volume of 7 micro liter ensures rapid turnover of tears at the ocular surface with a consequent removal of microorganisms.
Bacteria present in tears must also breach the defence provided by the proteins in the tear film such as lysozyme, lactoferrin, and transferrin. Furthermore,immunoglobulins such as IgA, IgG, IgE and IgM also offer hindrance to bacteria.
Any invading microorganism which is able to defeat all the above systems is still hampered in its quest to harm the cornea due to the followings
- Tight junctions b/w corneal epithelial cells to prevent migration of bacteria
- Sloughing of epithelial cells to shed infected cells before any further harm is caused to the rest of the cornea
- Active release of antibacterial factors from corneal epithelium
- A filter like barrier provided by basal lamina which stops entry into underling stroma
Contact lens use adversely affects a number of these defence mechanisms. A key reason for increase in corneal infections is the bio burden of microorganisms introduced to ocular surface by preventing the clearance of debris by blinking,when lens are inserted. It has also been postulated that level of fibronectin deceases during CL wear, which maximize the likelihood of bacterial attachment to corneal epithelium.
Therefore, an appropriate disinfecting system is essential to eliminate the risk of infection.
A rationale for cleaning contact lenses
There are two main reasons why to clean the lens prior to disinfection. First, a wide variety of intrinsic (proteins, lipids, mucins,
minerals from the eye) and extrinsic debris ( cosmetics, microorganisms and contaminants from external sources like hands) can adhere to the lens surface which can lead to lens distortion, discomfort, an unsightly cosmetic appearance and ocular pathology. Second, cleaning acts to enhance the disinfection process by reducing the level of microorganisms on lens. So, lens cleaning can alleviate these issues.
The Evolution of Soft Lens Care systems
Historically, the care of a soft contact lens was a complex and time consuming activity for the wearer. The various steps were divided into separate activities with a different bottle or tablet for each. This has changed over time for two reasons: First, introduction of multipurpose products and the second, commercial success of disposable and planned replacement of soft lenses acted as a powerful incentive for lens care industry to reduce the complexity of the lens care process as much as possible. Now a days, a great majority of soft lens wearer are prescribed MPS.
Solutions for Soft Lenses
The general principles for soft lens solutions are similar to those used for rigid lenses, but there are potentially more difficulties because of the possibility of interaction with the material.
Disinfection
It involves destruction of vegetative microorganisms with a limited effect on sporing microorganisms.
It is of two types.
- Thermal or heat
- Chemical or cold
Thermal Disinfection
Thermal disinfection is the least expensive and most effective disinfection system in the short term; however as the heat bakes on the deposits not cleaned off the lens, lens life is shortened and complications such as giant papillary conjunctivitis (GPC, also known as contact lens papillary conjunctivitis or CLPC) or red-eye reactions may arise from the deposited lens. As a disinfectant, thermal disinfection is effective against all forms of bacteria, including Pseudomonas,Acanthamoeba and the HIV virus.
The solutions used with thermal disinfection can be preservative-free for those patients sensitive to preserved solutions. Despite the advantages, the popularity of thermal disinfection has declined to the point where it is not used by contact lens wearers because of electrical requirements and the long-term problem of baked-on deposits. In addition, heat is contraindicated with lenses containing >55% water, and caution must be taken when switching a patient from another type of disinfection to thermal since it is not interchangeable with all systems. Manufacturers no longer support this type of disinfection with heat units for individual use.
Saline was extensively used for heat disinfection but is now mainly used for rinsing.
A solution of 0.9% saline is referred to as isotonic, having an overall sodium chloride concentration equivalent to that of human tears. A solution with higher salt concentration is hyper tonic and one with a lower concentration hypo tonic.
Normal (0.9%) saline may be either buffered or non buffered and is available in the following formats:
• Preserved in multi dose bottles
• Non preserved in unit dose form
• Non preserved in aerosol form – but no longer readily available.
• Drip-feed bags with one-way valve
The use of ultrasound systems for lens disinfection also proposed but it showed a limited effect.
Chemical Disinfection
Cold disinfection uses either preserved chemicals or non preserved oxidative systems.
After the problems associated with heat systems became known, alternate systems were required.
The problem that originally became apparent with chemical disinfection systems was the use of preservatives, such as thimerosal and chlorhexidine. Although both of these preservatives exhibit excellent preservative action, many patients were sensitive to them. Some patients still present with symptoms of dryness, itching, burning, injection, decreased wearing time, and discomfort. Changing the patient to a preservative-free, hydrogen peroxide care regimen alleviates the dry-eye symptoms.
Oxidative chemical disinfection
Oxidative systems are generally non preserved and use hydrogen peroxide or chlorine-based compounds as the disinfecting agent.
Hydrogen peroxide is a very effective disinfectant for a wide
range of bacteria and viruses for a relatively short exposure of
time.
Hydrogen peroxide 3%is used for 10 to 15 minutes. An oxidative reaction occurs whereby the hydrogen peroxide
molecule breaks down into free radical, which disrupts the cell
wall of the microorganisms. This free radical breaks into water
and oxygen by rinsing in normal saline and soaking in 2.5% sodium thiosulphate for 15 min.
0.005% to 0.006% is effective as preservative and 3% is effective
disinfectant.
Hydrogen peroxide disinfection system has following
advantages:
. It can penetrate deep into pores of lens matrix
. Has very good disinfecting property
. Does not need preservative as has acts as preservative on its
own
. It has some cleaning action by breaking down protein and
lipid bonds
. It is nontoxic if properly neutralized
It has following disadvantages:
. It may cause ocular toxicity if is not neutralized properly
. It is expensive
. More complex to use
. May effect parameters of FDA group IV lenses.
Chlorine
Chlorine releasing are long established disinfecting agents but no longer available due to availability of MPSs
Cleaning
This process should be done daily. Proper cleaning removes
90% of organisms, so the time the lens reaches the disinfection
step there is a significant reduction in microbial contamination.
Cleaners mostly contain—surfactant( polaxamer, sodium laurel sulphate), viscosity agent ( sodium hyaluronate, methyl cellulose etc),
chelating agent ( EDTA), buffer ( borate,phosphate,citrate) and preservative ( thiomersal, chlorhexidine, benzalkonium chloride).
There are some specialty cleaning agents like polymeric beads
which have abrasive cleaning agent or isopropyl alcohol for
dissolving lipids.
Enzyme tablets
Enzyme tablets are used for the removal of protein from the lens surface. Weekly cleaning is suggested, but patients with peroxide are able to use them less frequently.
Tablets are dissolved in saline or used in the actual disinfecting solution. The most common enzymatic cleaners are papain and pancreatin.
The overwhelming number of contact lens wearers who are fitted in disposable or frequent replacement soft lenses has almost eliminated the need for enzymatic cleaners. In addition,
many MPSs contain ingredients to aid in protein removal.
Lubrication or Re wetting
The use of lens lubricants or re wetting drops is optional; however, they may be beneficial in cases of dry eyes, foreign-body sensation, irritations, and for morning and evening use in overnight lens wear. Lens lubricants are used directly in the eye with or without the lenses.
Patients should not substitute artificial tears, GP lens lubricants, or ophthalmic medications for soft lens lubricants because the preservatives are not necessarily compatible with the soft lens materials, the possible result being lens discoloration and toxic reactions.
Common re wetting agents are polvinyl alcohol and hydroxypropyl methyl cellulose.
Multipurpose Solutions (MPSs)
These account for 90% of prescribed care regimens. By definition, these solutions don’t require the use of other auxiliary components in the lens care process.
Polyhexanide based MPS
It contains followings constituents
Preservative: Polyhexanide 1.0 ppm
Surfactant: Polaxamer
EDTA: 0.02%
Buffer: Phosphate
Wetting agent: Hydroxypropyl methyl cellulose
Polyquaternium-1 based MPS
It consists of followings
Preservative: Polyquad 10 ppm
Surfactant: Poloxamine
EDTA: 0.5%
Buffer: Borate
Wetting agent: Hydroxypropyl methyl cellulose
Rigid lens care systems
Disinfection and wetting
The majority of solutions used for wetting and soaking GP lenses combine several functions
into one solution. These solutions have four major functions.
1. To temporarily enhance the lens surface wettability.
2. To maintain the lens in a hydrated state similar to that achieved on the eye.
3. To disinfect the lens.
4. To act as a mechanical buffer between the lens and cornea.
The specific formulation of the ingredients in these solutions, especially the preservatives and wetting agents, is very important.
Preservatives are disinfectants and are capable of either killing microorganisms (bactericidal agents) or inhibiting their growth (bacteriostatic agents).
There are numerous preservatives currently in common use, all differing in their mode of action and effectiveness. The most common preservatives include benzalkonium chloride
(BAK), chlorhexidine, thimerosal, ethylenediamine tetraacetate (EDTA), polyaminopropyl biguanide (PAPB), polyquarternium-1 (polyquad), and benzyl alcohol.
Wetting solutions typically contain either polyvinyl alcohol (PVA) or a methyl cellulose derivative as a wetting agent.
PVA has several properties that make it a beneficial additive to GP lens solutions.
Cleaning
Cleaning solutions remove surface debris including lipids and mucus and enhance the disinfecting action of the soaking solution.
Some rigid lenses are cleaned with a separate solution to a disinfectant whereas mostly follow soft lens MPS.
Enzyme cleaning is arguably more important with rigid lenses than soft lenses because soft lenses are more frequently replaced.Tablets are dissolved in saline or one drop of liquid enzyme is placed
in the lens case with the disinfecting solution on a nightly basis.
Laboratory Cleaning
The use of laboratory-approved, extra-strength cleaners such as the Boston Lens Laboratory Cleaner (Bausch & Lomb) can be beneficial.
Plasma Cleaning
Plasma treatment for GP lenses removes residue from the manufacturing process and imparts an ultra-clean lens surface. This can enhance lens wetting and patient comfort and vision, particularly upon initial dispensing and early lens wear.
Re wetting
A solution that is used to re wet a GP lens surface while it is still on the eye should perform following functions:
1. Re wet the lens surface.
2. Stabilize the tear film.
3. Rinse away trapped debris.
4. Breakup loosely attached deposits.
Ideally, this solution should clean the lenses of any debris while re wetting them for an extended period. Essentially, these are surface-active chemical substances that increase the spreading and penetrating properties of a liquid by lowering its surface tension. Because the
key to re wetting a GP lens is contact time, PVA often is added to increase the length of contact time.
Guidelines for patients
- Personal hygiene is the mainstay of lens care. Hands should be meticulously washed and dried with a lint free towel before and insertion and removal of lens. Fingernails must be clipped and cleaned. The use of soaps with a hand or cold cream should be avoided as these may contaminate the lens.
- Employ only recommend and follow the methods of insertion and removal as recommended by the practitioner.
- Before insertion, always be careful if the lens is inside out. Hold the lens b/w index finger and thumb and pinch it at the base. If the edges curl inside, lens is in a correct way. If the edges curl outside, lens is inside out. This is called TACO test. Some manufacturers also mention inside out mark or logo at the lens edge.
- Hard lenses are kept in a dry state in a simple preferably flat case to fit in a pocket or purse. Case needs to be cleaned once in a week and replaced after 1-3 months. Soft lenses are stored in a wet state in a hydrating kit after thorough cleaning. Kit should be filled with enough solution to fill the lens. Lens case should be cleaned twice a week and changed after 1-3 months. Take care of lenses mixing and always keep right and left lenses in their respective portions.
- Clean and disinfect lenses whenever it is required. Don’t use tap water for both hard and soft lenses as it is not sterile.
- If lens is dropped at the floor, don’t slide it along the floor as it may get scratched or may produce electrical charge which could attract foreign body. Just wet the lens with lens solution and pick it up gently. Avoid using saliva to wet the lens.
- Solutions should not be reused and one needs to careful while purchasing solutions as MPS of hard and soft lenses look similar and one may mistakenly be handled the wrong solution. Also always check expiry date.
- Lenses are applied and removed first before make eye make up and when make up is cleaned. Maskara should be oil free and water proof. Opt for cream based eye shadows instead of powder or glitter eye shadow. Refrain from applying eyeliner on waterline with your lenses in the eyes. However, if eyeliner is must, invest in a kajal pencil which is smudge proof and sharpened in order to avoid getting any of it on lenses.
- In men, problem in contamination with hair or occupational oils and tobacco stains.
- Children are usually unaware of need of lens care regimen. So parents need to be extra vigilant and supervise their children and set a strict routine for the child to follow.
Guidelines for Practitioners
Soft Lenses
Educating patients about the wearing, handling, and care of soft lenses should be considered as serious and important to the success of soft lens wear as the fit of the lenses. Patient noncompliance and absence of knowledge are primary causes of soft complications, which may be a result of the patient’s lack of concern, the practitioner’s lack of seriousness, or both. When patient education becomes secondary, taught by staff members who are less than knowledgeable and with little emphasis, a lack of importance is perceived by the patients that carries over into their lens care routine. Typically, four practitioner factors contribute to patient noncompliance: poor instructions, no instructions, poor example, and overloading the patient with information. Every office should provide the patient with written information pertaining to insertion and removal, lens care, and lens handling.
Hard Lenses
The key to teaching patients to handle GP lenses successfully is reassurance. No matter how frustrating it is to the person performing the instruction, that feeling of frustration must not
be conveyed to the patient. The instructions must be provided slowly and one at a time.The patient should not have the perception that the eye care provider or assistants have lost confidence in the patient’s ability to learn how to handle the lenses properly; otherwise, feelings of failure and may arise possibly leading to the attitude that contact lenses will never be worn again. Conversely, if the patient feels confident about handling
the lenses, a perception of satisfaction and success is often present. A minimum of three successful insertions and removals is recommended, although the number depends on how confident the patient feels.
It is important for both practitioner and staff members to set a good example by washing hands before handling lenses in the office with a view to urge patients to do likewise. Use of an antimicrobial soap is recommended to prevent the transfer of pathogens from hand to lenses during the insertion and removal process. Hands should be washed for total of 30 seconds using two pumps of an antimicrobial pump prior to handling the lenses.