Light is a form of energy whose interaction with retina gives the sensation of sight. Thus, light is the visible portion of the electromagnetic radiation spectrum. It lies between ultraviolet and infrared portion, from 400 nm at the violet and end of spectrum to 700 nm at the red end. This wavelength means a frequency range of roughly 430-750 terahertz (THz). The white light consists of seven colors denoted by ‘VIBGYOR’ (violet, indigo, blue, green, yellow, orange and red).
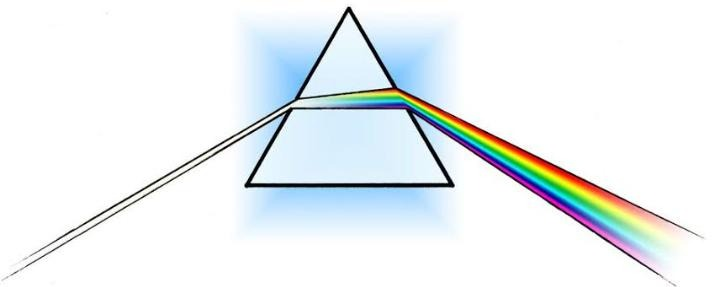
Light or visible light is electromagnetic radiation within the portion of the electromagnetic spectrum that is perceived by the human eye. A triangular prism dispersing a beam of white light. The longer wavelengths (red) and the shorter wavelengths (blue) are separated.
The primary properties of visible light are intensity, propagation-direction, frequency or wavelength spectrum and polarization. Its speed in a vacuum, 299 792 458 metres a second (m/s), is one of the fundamental constants of nature, as with all types of electromagnetic radiation (EMR), light is found in experimental conditions to always move at this speed in a vacuum. In physics, the term ‘light’ sometimes refers to electromagnetic radiation of any wavelength, whether visible or not.
In this sense, gamma rays, X-rays, microwaves and radio waves are also light. Like all types of electromagnetic radiation, visible light propagates as waves. However, the energy imparted by the waves is absorbed at single locations the way particles are absorbed. The absorbed energy of the electromagnetic waves is called a photon and represents the quanta of light. When a wave of light is transformed and absorbed as a photon, the energy of the wave instantly collapses to a single location and this location is where the photon “arrives”. This is what is called the wave function collapse. This dual wave-like and particle-like nature of light is known as the wave–particle duality. The study of light, known as optics, is an important research area in modern physics.
The main source of light on Earth is the Sun. Historically, another important source of light for humans has been fire, from ancient campfires to modern kerosene lamps. With the development of electric lights and power systems, electric lighting has effectively replaced firelight.
Electromagnetic spectrum and visible light: Generally, EM radiation (the designation “radiation” excludes static electric, magnetic and near fields), or EMR, is classified by wavelength into radio waves, microwaves, infrared, the visible spectrum that we perceive as light, ultraviolet, X-rays and gamma rays.
The behavior of EMR depends on its wavelength. Higher frequencies have shorter wavelengths and lower frequencies have longer wavelengths. When EMR interacts with single atoms and molecules, its behavior depends on the amount of energy per quantum it carries.
EMR in the visible light region consists of quanta (called photons) that are at the lower end of the energies that are capable of causing electronic excitation within molecules, which leads to changes in the bonding or chemistry of the molecule. At the lower end of the visible light spectrum, EMR becomes invisible to humans (infrared) because its photons no longer have enough individual energy to cause a lasting molecular change (a change in conformation) in the visual molecule retinal in the human retina, which change triggers the sensation of vision.
There exist animals that are sensitive to various types of infrared, but not by means of quantum-absorption. Infrared sensing in snakes depends on a kind of natural thermal imaging, in which tiny packets of cellular water are raised in temperature by the infrared radiation. EMR in this range causes molecular vibration and heating effects, which is how these animals detect it.
Above the range of visible light, ultraviolet light becomes invisible to humans, mostly because it is absorbed by the cornea below 360 nm and the internal lens below 400 nm. Furthermore, the rods and cones located in the retina of the human eye cannot detect the very short (below 360 nm) ultraviolet wavelengths and are in fact damaged by ultraviolet. Many animals with eyes that do not require lenses (such as insects and shrimp) are able to detect ultraviolet, by quantum photon-absorption mechanisms, in much the same chemical way that humans detect visible light.
Various sources define visible light as narrowly as 420–680 nm to as broadly as 380–800 nm. Under ideal laboratory conditions, people can see infrared up to at least 1,050 nm; children and young adults may perceive ultraviolet wavelengths down to about 310–313 nm.
Plant growth is also affected by the color spectrum of light, a process known as photomorphogenesis.
Speed of light: The speed of light in a vacuum is defined to be exactly 299 792 458 m/s (approx. 186,282 miles per second). The fixed value of the speed of light in SI units results from the fact that the metre is now defined in terms of the speed of light. All forms of electromagnetic radiation move at exactly this same speed in vacuum.
Different physicists have attempted to measure the speed of light throughout history. Galileo attempted to measure the speed of light in the seventeenth century. An early experiment to measure the speed of light was conducted by Ole Rømer, a Danish physicist, in 1676. Using a telescope, Rømer observed the motions of Jupiter and one of its moons, Io. Noting discrepancies in the apparent period of Io’s orbit, he calculated that light takes about 22 minutes to traverse the diameter of Earth’s orbit. However, its size was not known at that time. If Rømer had known the diameter of the Earth’s orbit, he would have calculated a speed of 227 000 000 m/s.
Another more accurate measurement of the speed of light was performed in Europe by Hippolyte Fizeau in 1849. Fizeau directed a beam of light at a mirror several kilometers away. A rotating cog wheel was placed in the path of the light beam as it traveled from the source, to the mirror and then returned to its origin. Fizeau found that at a certain rate of rotation, the beam would pass through one gap in the wheel on the way out and the next gap on the way back. Knowing the distance to the mirror, the number of teeth on the wheel and the rate of rotation, Fizeau was able to calculate the speed of light as 313 000 000 m/s.
Léon Foucault carried out an experiment which used rotating mirrors to obtain a value of 298 000 000 m/s in 1862. Albert A. Michelson conducted experiments on the speed of light from 1877 until his death in 1931. He refined Foucault’s methods in 1926 using improved rotating mirrors to measure the time it took light to make a round trip from Mount Wilson to Mount San Antonio in California. The precise measurements yielded a speed of 299 796 000 m/s.
The effective velocity of light in various transparent substances containing ordinary matter, is less than in vacuum. For example, the speed of light in water is about 3/4 of that in vacuum.
Two independent teams of physicists were said to bring light to a “complete standstill” by passing it through a Bose–Einstein condensate of the element rubidium, one team at Harvard University and the Rowland Institute for Science in Cambridge, Massachusetts and the other at the Harvard–Smithsonian Center for Astrophysics, also in Cambridge. However, the popular description of light being “stopped” in these experiments refers only to light being stored in the excited states of atoms, then re-emitted at an arbitrary later time, as stimulated by a second laser pulse. During the time it had “stopped” it had ceased to be light.
Optics: An example of refraction of light. The straw appears bent, because of refraction of light as it enters liquid (the water, in this case) from air.
A cloud illuminated by sunlight Refraction is the bending of light rays when passing through a surface between one transparent material and another. It is described by Snell’s Law:
{\display style n_{1}\sin \theta {1}=n{2}\sin \theta _{2}\ .}n_1\sin\theta_1 = n_2\sin\theta_2\ .
where θ1 is the angle between the ray and the surface normal in the first medium, θ2 is the angle between the ray and the surface normal in the second medium and n1 and n2 are the indices of refraction, n = 1 in a vacuum and n > 1 in a transparent substance.
When a beam of light crosses the boundary between a vacuum and another medium, or between two different media, the wavelength of the light changes, but the frequency remains constant. If the beam of light is not orthogonal (or rather normal) to the boundary, the change in wavelength results in a change in the direction of the beam. This change of direction is known as refraction.
The refractive quality of lenses is frequently used to manipulate light in order to change the apparent size of images. Magnifying glasses, spectacles, contact lenses, microscopes and refracting telescopes are all examples of this manipulation.
Light sources: “Light source” redirects here. For the solar energy developer named Light source, see Light source Renewable Energy.
There are many sources of light. A body at a given temperature emits a characteristic spectrum of black-body radiation. A simple thermal source is sunlight, the radiation emitted by the chromosphere of the Sun at around 6,000 kelvins (5,730 degrees Celsius; 10,340 degrees Fahrenheit) peaks in the visible region of the electromagnetic spectrum when plotted in wavelength units and roughly 44% of sunlight energy that reaches the ground is visible. Another example is incandescent light bulbs, which emit only around 10% of their energy as visible light and the remainder as infrared. A common thermal light source in history is the glowing solid particles in flames, but these also emit most of their radiation in the infrared and only a fraction in the visible spectrum.
The peak of the black-body spectrum is in the deep infrared, at about 10 micro metre wavelength, for relatively cool objects like human beings. As the temperature increases, the peak shifts to shorter wavelengths, producing first a red glow, then a white one and finally a blue-white color as the peak moves out of the visible part of the spectrum and into the ultraviolet. These colors can be seen when metal is heated to “red hot” or “white hot”. Blue-white thermal emission is not often seen, except in stars (the commonly seen pure-blue color in a gas flame or a welder’s torch is in fact due to molecular emission, notably by CH radicals (emitting a wavelength band around 425 nm and is not seen in stars or pure thermal radiation).
Atoms emit and absorb light at characteristic energies. This produces “emission lines” in the spectrum of each atom. Emission can be spontaneous, as in light-emitting diodes, gas discharge lamps (such as neon lamps and neon signs, mercury-vapor lamps, etc.) and flames (light from the hot gas itself—so, for example, sodium in a gas flame emits characteristic yellow light). Emission can also be stimulated, as in a laser or a microwave maser.
Deceleration of a free charged particle, such as an electron, can produce visible radiation: cyclotron radiation, synchrotron radiation and bremsstrahlung radiation are all examples of this. Particles moving through a medium faster than the speed of light in that medium can produce visible Cherenkov radiation. Certain chemicals produce visible radiation by chemoluminescence. In living things, this process is called bioluminescence. For example, fireflies produce light by this means and boats moving through water can disturb plankton which produce a glowing wake.
Certain substances produce light when they are illuminated by more energetic radiation, a process known as fluorescence. Some substances emit light slowly after excitation by more energetic radiation. This is known as phosphorescence. Phosphorescent materials can also be excited by bombarding them with subatomic particles. Cathodoluminescence is one example. This mechanism is used in cathode ray tube television sets and computer monitors.
Light pressure: Usually light momentum is aligned with its direction of motion. However, for example in evanescent waves momentum is transverse to direction of propagation.
Wave theory: To explain the origin of colors, Robert Hooke (1635–1703) developed a “pulse theory” and compared the spreading of light to that of waves in water in his 1665 work Micro graphia (“Observation IX”). In 1672 Hooke suggested that light’s vibrations could be perpendicular to the direction of propagation. Christiaan Huygens (1629–1695) worked out a mathematical wave theory of light in 1678 and published it in his Treatise on light in 1690. He proposed that light was emitted in all directions as a series of waves in a medium called the luminiferous either. As waves are not affected by gravity, it was assumed that they slowed down upon entering a denser medium.
Electromagnetic theory: A 3–dimensional rendering of linearly polarized light wave frozen in time and showing the two oscillating components of light; an electric field and a magnetic field perpendicular to each other and to the direction of motion (a transverse wave).
In 1845, Michael Faraday discovered that the plane of polarization of linearly polarized light is rotated when the light rays travel along the magnetic field direction in the presence of a transparent dielectric, an effect now known as Faraday rotation. This was the first evidence that light was related to electromagnetism. In 1846 he speculated that light might be some form of disturbance propagating along magnetic field lines. Faraday proposed in 1847 that light was a high-frequency electromagnetic vibration, which could propagate even in the absence of a medium such as the ether.
Faraday’s work inspired James Clerk Maxwell to study electromagnetic radiation and light. Maxwell discovered that self-propagating electromagnetic waves would travel through space at a constant speed, which happened to be equal to the previously measured speed of light. From this, Maxwell concluded that light was a form of electromagnetic radiation: he first stated this result in 1862 in On Physical Lines of Force. In 1873, he published A Treatise on Electricity and Magnetism, which contained a full mathematical description of the behavior of electric and magnetic fields, still known as Maxwell’s equations. Soon after, Heinrich Hertz confirmed Maxwell’s theory experimentally by generating and detecting radio waves in the laboratory and demonstrating that these waves behaved exactly like visible light, exhibiting properties such as reflection, refraction, diffraction and interference. Maxwell’s theory and Hertz’s experiments led directly to the development of modern radio, radar, television, electromagnetic imaging and wireless communications.
In the quantum theory, photons are seen as wave packets of the waves described in the classical theory of Maxwell. The quantum theory was needed to explain effects even with visual light that Maxwell’s classical theory could not (such as spectral lines).
Quantum theory: In 1900 Max Planck, attempting to explain black-body radiation, suggested that although light was a wave, these waves could gain or lose energy only in finite amounts related to their frequency. Planck called these “lumps” of light energy “quanta” (from a Latin word for “how much”). In 1905, Albert Einstein used the idea of light quanta to explain the photoelectric effect and suggested that these light quanta had a “real” existence. In 1923 Arthur Holly Compton showed that the wavelength shift seen when low intensity X-rays scattered from electrons (so called Compton scattering) could be explained by a particle-theory of X-rays, but not a wave theory. In 1926 Gilbert N. Lewis named these light quanta particles photons.
Eventually the modern theory of quantum mechanics came to picture light as (in some sense) both a particle and a wave and (in another sense), as a phenomenon which is neither a particle nor a wave (which actually are macroscopic phenomena, such as baseballs or ocean waves). Instead, modern physics sees light as something that can be described sometimes with mathematics appropriate to one type of macroscopic metaphor (particles) and sometimes another macroscopic metaphor (water waves), but is actually something that cannot be fully imagined. As in the case for radio waves and the X-rays involved in Compton scattering, physicists have noted that electromagnetic radiation tends to behave more like a classical wave at lower frequencies, but more like a classical particle at higher frequencies, but never completely loses all qualities of one or the other. Visible light, which occupies a middle ground in frequency, can easily be shown in experiments to be describable using either a wave or particle model, or sometimes both.
In February 2018, scientists reported, for the first time, the discovery of a new form of light, which may involve polaritons, that could be useful in the development of quantum computers.
Use for light on Earth: Sunlight provides the energy that green plants use to create sugars mostly in the form of starches, which release energy into the living things that digest them. This process of photosynthesis provides virtually all the energy used by living things. Some species of animals generate their own light, a process called bioluminescence. For example, fireflies use light to locate mates and vampire squid use it to hide themselves from prey.