Introduction:
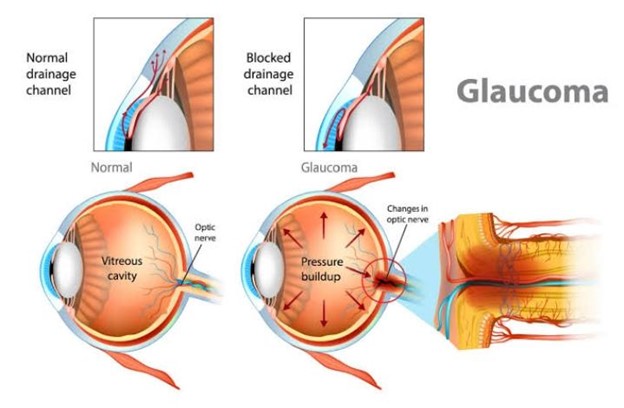
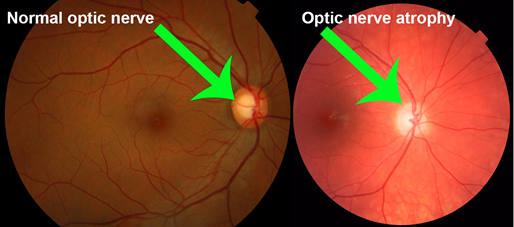
Glaucoma is a mixed group of eye disorders sharing common features which typically includes optic disc cupping and visual field loss, thereby more accurately defined as an optic neuropathy. Optic nerve damage results in progressive retinal ganglionic cell loss
and irreversible blindness, if not treated.1 Traditionally, glaucoma has been characterized by the existence of raised intraocular pressure (IOP), however nerve damage may occur in the presence of values within the statistically normal range between Both types can be further divided into primary versus secondary, and congenital versus acquired. Glaucoma has a global prevalence of 3.54% in those aged between 40 and 80 years, and is the second leading cause of irreversible blindness, only superseded by cataracts. Open angle glaucoma is the highest in Africa (4.2%),
while closed angle glaucoma is more prevalent in Asia (1.09%).
Open angle glaucoma:
In open angle glaucoma, the trabecular meshwork undergoes morphological changes over time, resulting in progressive impairment of drainage although it remains anatomically open. Open angle glaucoma most often affects both eyes, but asymmetric involvement is not uncommon. Primary open angle glaucoma (POAG) is the most common type and accounts for 74% of all glaucoma cases. It is estimated that 57.5 million people (3.05%) worldwide suffer from POAG.4 Prevalence among Black populations is the highest, with 2.1% at age 40 and 12.2% at age 80 being reported. The incidence in Asian populations (0.8% at age 40 and 4.3% at age 80), and Caucasians (0.4% at 40 years and 5.3% at 80 years) suggests an ethno-genetic disease pattern. Although the exact cause of POAG remains unknown, more than fifty risk factors and secondary causes have been identified. Some of these include diabetes mellitus, migraine headache, cardiovascular disease, asthma or COPD (inhaled anticholinergics and glucocorticosteroids), myopia, advanced age and a family history of first-degree relatives with the same condition. Disease progression leads to the formation of scar tissue between the iris and trabecular meshwork and will ultimately result in closing off the anterior chamber. The consequent rise in IOP is transmitted to the optic disc and is eventually responsible for atrophy and nerve fiber damage. Owing to the slow progression of the disease, POAG remains asymptomatic until central vision is affected. It does not present with headache, eye pain or loss of visual acuity. Diagnosis is usually incidental during routine funduscopic or optometry examinations. A cup : disc ratio of 0.6 (glaucomatous cupping), and central corneal thickness in the presence of risk factors is suggestive of glaucoma, and requires ophthalmological referral . The progression from POAG to Chronic Angle-Closure Glaucoma (CACG), requires lifelong treatment. Official general population screening policies do not exist and are not cost-effective. Measuring IOP has a sensitivity of only 47.1%, implying that nearly half of patients with POAG will have IOP below 22 mmHg. However, patients with
risk factors are encouraged to undergo annual ophthalmological examinations and additional IOP measurements.
Closed angle glaucoma :
With closed angle glaucoma, a primary hypermetropic anatomical defect (such as weakened ciliary muscles, naturally small eyes, narrow angle or thick lens) in the absence of an identifiable secondary cause, results in the pupil compressing the drainage canal between the iris and cornea. Compression and narrowing of the canal reduces or obstructs the outflow of aqueous humour in passing through the pupil, therefore accumulating in the anterior chamber. Primary angle closure glaucoma (PACG) is less common than POAG and accounts for approximately a third of all cases, with a global incidence of 0.5%. Symptoms of closed angle glaucoma depend on the degree of angle closure and in some instances may resolve spontaneously; in which case it may become chronic. In established disease, vision loss is severe and permanent. Secondary causes of angle-closure glaucoma include masses or hemorrhage in the posterior segment of the eyeball or the presence of a fibrovascular membrane (neovascular glaucoma) over the anterior chamber angle. Risk factors for
angle closure glaucoma include older age (> 60 years), female gender, Asian ethnicity, hyperopia and a positive family history. Attacks occur more frequently at night when diminished light cause mydriasis and narrowing of the angle.12 Unexpected closure (acute angle closure glaucoma, AACG) of the anterior chamber angle due to pupillary block may result in a sudden rise in IOP. It constitutes a medical emergency, since permanent blindness and irreversible damage to the optic nerve may occur. Acute attacks are characterized by intermittent symptoms such as severe eye pain, nausea and vomiting, loss of vision, halos around lights, unilateral red tearing eye, oval shaped non-reactive pupil, a cloudy cornea and the loss of red reflex. In addition, it is estimated that prescribed, or over-the-counter pharmacological agents are responsible for at least 30% of acute angle closure glaucoma. These include antihistamines, cholinergic and anticholinergic agents, adrenergic agents, antidepressants, sulphonamides, anticoagulants and glucocorticosteroids. below summaries the mechanism responsible
for causing AACG.
Treatment :
The goal in treating POAG is to establish and maintain the intraocular pressure at a range where visual field loss will have the least negative impact on the patient’s perceived visual disability. “Various studies have shown that visual field loss in glaucoma has been associated with a significant decrease in a person’s visionrelated quality of life. This is evident in activities requiring optimal vision, such as dark adaptation, avoiding unintentional bumping into objects and outdoor mobility. Determining the target intraocular pressure needs to be individualized for each patient, since the measured pressure at diagnosis could be within the normal range of 8 to 21 mmHg in 30–50% of patients.16 Aiming for pressures within these ranges will contribute towards optic nerve damage and further progression. It is generally accepted that patients with early disease benefit from a reduction of at least 20% in the baseline value. A reduction of 30% is recommended in individuals with more advanced features of POAG.17 Target IOP should continuously be re-calculated during monitoring and follow-up assessment of visual fields and disc and nerve fiber examinations. Reducing the IOP, however, does not guarantee that progression will not transpire. The choice of therapy depends on the relative risks and benefits of available modalities, which include pharmacological treatment, laser therapy or incisional surgery. Factors such as cost, dosing, patient’s age, the presence of comorbidities (asthma or COPD), pregnancy, adherence to treatment and the degree of optic disc damage should be considered in choosing an appropriate treatment regimen. In treating AACG, the primary goal is to make the correct diagnosis to prevent permanent loss of vision. This is achieved by rapid lowering of the IOP and reversal of the angle closure. Empiric treatment with topical and systemic aqueous humour suppressants (β blockers and acetazolamide) reduces the IOP, followed by pilocarpine to reverse the pupil block. In the absence of
available trials comparing medical options for treatment of AACG, the initial management usually consists of administering one drop of 0.5% timolol, 1% apraclonidine and 2% pilocarpine one minute apart. This is followed by intravenous acetazolamide or mannitol. Eye pressure should be checked every hour until specialist ophthalmological care can be provided. Pharmacological management : Pharmacological management is the most common initial intervention in lowering the IOP, although laser trabeculoplasty and surgery are frequently used to slow the disease progression. Surgical treatment has no advantage over pharmacological management in visual field protection, however surgical interventions attain target IOP in 46% of cases compared to 35% in those being treated medically. Several agents are available and the primary choice of drug depends on the degree of pressure lowering which needs to be achieved. It is recommended to initiate treatment on one type of medication. If an inadequate response in lowering the IOP is observed, the dose may be adjusted, or a second agent may be added. If the first agent fails to demonstrate any measurable clinical response, it should be removed from the regimen and be replaced with an alternative drug. Pharmacological agents used to lower the IOP include prostaglandin analogs, carbonic anhydrase inhibitors, α2 adrenergic agonists, cholinergic agonists, β blockers and hyperosmotic agents. Adequate treatment of glaucoma requires a high level of adherence. It is estimated that 45% of patients will default on chronic treatment, if they are not appropriately followed up and counselled regarding dosage instructions and possible side effects.
Prostaglandin receptor analogs :
Latanoprost, travoprost and bimatoprost are prostaglandin F2 agonists, which increases the uveoscleral outflow of aqueous humour. It is considered the most potent group of topical ocular hypotensive agents currently available and may lower baseline IOP by 25–35%.23 There are few associated systemic side effects and mostly relates to headaches. In addition, latanoprost may cause joint/muscle pain and flu-like symptoms. Local adverse effects include blurred vision, conjunctival hyperaemia, eye discomfort, permanent iridial pigmentation, macular edema and thickening of the eyelashes.
Carbonic anhydrase inhibitors :
Acetazolamide, dorzolamide and brinzolamide are sulphonamide derivatives which reduces aqueous humour production independent to its diuretic mode of action. Acetazolamide is administered systemically in the form of oral tablets or intravenous infusion, and additionally reduces the rate of aqueous inflow up to 50%. Its usefulness is limited to the management of acute closed angle glaucoma due to unfavourable systemic side effects which may include paresthesia, nausea, diarrhoea, loss of
appetite and dose related systemic acidosis. Topical application of dorzolamideand brinzolamide demonstrates an improvement in ocular blood flow and long-term preservation of visual field. An average reduction of 15–20% in baseline IOP can be expected. Side effects include ocular irritation, dry eyes, blurred vision, eyelid inflammation and a bitter taste.
Alpha 2 adrenergic agonists :
Brimonidine is a selective α2 receptor agonist. It initially reduces aqueous humour production followed by a subsequent increase in the uveoscleral outflow, therefore proving to be useful in preventing a rise in IOP prior to anterior segment laser surgery. Brimonidine reduces baseline IOP with approximately 20%. Apraclonidine reduces the formation of aqueous humour but has no effect on the facilitation of outflow. It has affinity for both α1 and α2 adreno-receptors and are used to prevent postsurgical elevation of IOP after anterior segment laser therapy. Apraclonidine reduces baseline
IOP by 30% and is additionally used as adjuvant therapy in resistant cases of glaucoma. Systemic side effects of the alpha agonists are related to the central nervous system (headache, fatigue, insomnia, depression) and respiratory system. Caution should be taken in patients with cerebral or coronary insufficiency, postural hypotension and renal or hepatic failure. Hypersensitivity reactions, ocular irritation, eyelid edema, foreign body sensation and dry eyes are common with topical use.
Cholinergic agonists :
Pilocarpine is the only available cholinergic agent in the management of acute closed- angle glaucoma registered in South Africa. Administration results in the increase in aqueous humour outflow by contraction of the ciliary muscle and constriction of the pupil. Poorly tolerated side effects, such as ciliary muscle spasm, myopia and decreased vision limits its usefulness.
Beta Blockers :
Although prostaglandin receptor agonists are the most potent agents in reducing the IOP, beta blockers are regarded as the first choice in treating open angle glaucoma. Individual agents differ in their ability to lower IOP, with betaxolol achieving a 15% decrease, compared to 20–25% with timolol and levobunolol respectively. Betaxolol is, however, more cardioselective (β1) and therefore has fewer pulmonary side effects
compared to timolol and levobunolol (which are non-selective antagonists on β1 and β2 receptors). The mechanism involves a reduction of aqueous humour production and does not cause miosis or accommodation disturbances compared to cholinergic agents. The most common adverse effects are ocular irritation and dry eyes. All the β blockers (including the cardioselective agents) are contraindicated in patients with asthma, COPD and bradycardia, unless alternative treatment is unavailable.
Combination agents :
Several combination agents are available in the treatment of glaucoma. Most of these combinations include timolol, since β blockers are still considered first line therapy in open-angle glaucoma and ocular hypertension. Fixed-dose combination therapy is more efficacious than its individual components, but still less efficacious than its respective unfixed combinations. It provides for simplified treatment regimes and enhances compliance. Fixed dose combinations containing prostaglandins have a lower risk in causing hyperemia. Combination of timolol with dorzolamide is superior in visual field preservation compared to timolol and brinzolamide.26 Combination preparations with brimonidine have less visual disturbances, taste alterations and ocular allergic reactions compared to the single ingredient product.
Cannabinoids :
With the recent attention cannabis has been receiving in the management of pain and other cancer related conditions, it is important to remember the favorable effect of its active ingredient, Δ9-tetrahydrocannabinol (THC), in reducing ocular blood flow. Studies from the 1970s have reported a 30% reduction in the IOP by inhalation or intravenous administration of THC. The use is, however, limited owing to the cardiovascular (increased heart rate and decreased blood pressure) and neurological (tolerance and addiction) side effects. Topical application does not produce a significant clinical effect. The use of synthetic cannabinoids may provide a basis for potential future treatment strategies.