Introduction –
Optical interventions for controlling myopia have an extensive history, with early clinical studies largely
based around spectacles aimed at altering the near visual experience. Clinical studies involving contact
lens-based treatments are largely limited to the 21st century, with studies demonstrating optical defocus-
driven regulation of eye growth in animal models helping to reawaken interest in, and drive new optical
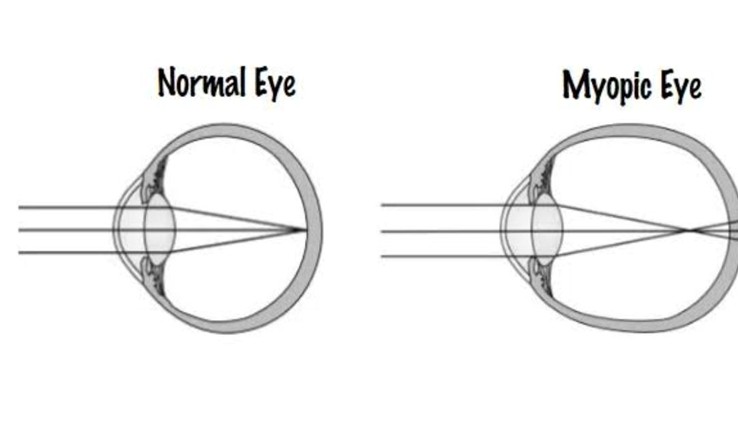
approaches to myopia control. Specifically, as demonstrated first in young chicks, imposed myopic
defocus is known to slow eye growth, whereas the converse is true for hyperopic defocus (i.e., eye
growth accelerates).This report summarizes the results from clinical studies using spectacles, contact
lenses, and orthokeratology (OK). The evidence contained in relevant published studies has been
evaluated and recommendations for using optical strategies for myopia control provided, based on the
quality of reported results and the evidence.
Spectacles –
The utility of using spectacle lenses for slowing myopia progression has many advantages over other
forms of myopia management, as they are easy to fit, are mostly well accepted and tolerated, are
affordable by most, and are minimally invasive. The various spectacle lens-based approaches aimed at
slowing the progression of myopia include both standard and customized single vision (SV) lens designs,
as well as bifocal and progressive spectacle lenses.
There is equivocal evidence concerning whether full
correction with SV spectacles causes faster myopic progression than full correction with soft contact
lenses. The evidence would suggest that if that is the case then the difference is likely clinically irrelevant.
Under correction With Spectacles-
Under correction to slow the progression of myopia has been in practice for many years and was originally
considered to slow the progression of myopia by reducing the accommodative demand during near tasks.
The accumulating reports of slowed eye growth in response to experimentally imposed myopic
defocus in animal models," also Ied to paralels bcing dravn with the myopic defocus experienced during
distance tasks with under correction, and thus speculation about this potential additional benefit.
An early nonrandomized trial of under correction, conduct-ed in 1960s, found this treatment to slow the
progression of myopia. More recently (since 2000), well-designed, randomized controlled trials (RCTS)
examining under correction for distance (by +0.50 to +0.75 diopters [D]) over 1.5 to 2.0 years found this
treatment to either increase myopia progression or have no benefit, when compared with myopia
progression in fully corrected SV spectacle wearers. Although
all trials involved relatively young children at an age when progression is common, the trials were only
small to moderate in size. However, the latter weakness does not explain the consistent trend of faster
progression in under corrected eyes observed in some studies. Nonetheless, although another larger,
albeit nonrandomized trial also found no significant difference between comparable treatment groups,
curiously, myopia progression significantly decreased with increasing under correction. The latter trend is
also consistent with results from a recent study comparing myopia progression in uncorrected and fully
corrected 12-year-old children; this study found slower progression in the former group, the latter effect
increasing with the amount of under correction. If The possibility that the lack of sharp distance vision with
under-correction strategies may lead to behavioral changes, such as reduced outdoor activities in some
children, thereby favoring myopia progression, warrants investigation, although the contrasting study
outcomes suggest additional factors are at play.
Peripheral Defocus-Correcting Lenses-
Findings from animal studies, including monkeys," offer strong evidence for contributions by the
peripheral retina to eye growth regulation and refractive development. In addition, a number of studies
have reported relative peripheral hyperopia in myopic eyes when fully corrected with SV spectacles.
Thus, it has been
hypothesized that the hyperopic defocus experienced by the retinal periphery may drive further axial
elongation.
Three novel spectacle lens designs aimed at reducing the
relative peripheral defocus were tested in an RCT designed to evaluate this notion. The results were
generally disappointing, with no significant differences in myopia progression between the groups
observed. In subgroup analysis, one of the lens designs (Type III) that was specific to right and left eyes
demonstrated a small benefit (of 0.25 D),compared with SV spectacles in younger children with parental
myopia. Likewise, a recent trial involving Japanese children found no benefit of the MyoVision lens, a
positively aspherized design, " and in a further test of this treatment approach, no benefit was found by
combining a peripheral defocus correction with a progressive addition zone for near work.
Atropine-
Based on changes in spherical equivalent refractive error as the outcome measure all studies have
shown that atropine slows myopia progression. Bedrossian (1971) in an early study of 150 children aged
7 to 13 years reported no myopia progression in 75% of eyes treated daily with 1% atropine over a 1-year
period compared with only 3% of controls. Similarly another early study by Gimbel (1973)103 in which
279 children received 1% atropine over 3 years reported a 66% reduction in myopia progression
compared with that of 572 controls (-0.41 vs. -1.22 D).
The first two randomized controlled trials of atropine, both published in the 1990s, also reported very
good control over myopia progression in children, with reductions exceeding 60% reported for the
highest, 1% concentration. In the first randomized controlled trial by Yen and colleagues (1989), 247
children aged 6 to 14 years received either topical 1% atropine, 1% cyclopentolate, or saline drops over a
1-year period. They reported 76% and 36% reductions in myopia progression in the groups treated with
atropine and cyclopentolate, respectively, compared with the group treated with saline, although
unfortunately, there was a large loss to follow-up (61%). In the second randomized controlled trial by Shih
and colleagues (1999), 200 children aged 6 to 13 years were treated with 0.5%, 0.25%, or 0.1% atropine
over a 2-year period; reported reductions in myopia progression were 61%, 49%, and 42%, respectively,
compared with children treated with 0.5% tropicamide as the control treatment.
The ATOM1 and 2 studies, which were performed between 1996 and 2013, involved 400 children, aged 6
to 12 years, randomized in each case, to atropine 1% and placebo in a 1:1 ratio in ATOM1, and to 0.5%,
0.1%, and 0.01% atropine in a 2:2:1 ratio in ATOM2. Both trials involved a 2-year treatment period. On
entering the studies, children had low to moderate myopia; baseline spherical equivalent refractive errors
ranged between -1.0 and 6.0 D in ATOM1, and between -2.0 and-6.0 D in ATOM2. Overall, the profiles of
the participants in these two trials were very similar, although slightly younger, with lower myopia in the
first compared with the second trial (9.2 vs. 9.6 years; -3.4 vs. -4.7 D). 116,118 The reported mean
progression rates for these trials were -0.2, -0.3, -0.4, and -0.5 D for the four atropine groups (1%, 0.5%,
0.1%, and 0.01%) compared with -1.2 D in the placebo group, 115-119 amounting to reductions in myopia
progression compared with the latter group of approximately 80%, 75%, 67%, and 58%, respectively.
Loss to follow-up over the 2-year treatment periods was 13% and 11% for ATOM1 and ATOM2,
respectively.
Analysis of the changes in the ATOM1 study, year by year, revealed a hyperopic shift in the 1% atropine
group of +0.03 versus -0.79 D in the control arm. 118 The comparable values for the 0.5%, 0.1%, and
0.01% atropine treatment groups included in ATOM2 are -0.17, -0.31, and -0.43 D respective-ly. Thus,
myopia progression rates appear to directly reflect the atropine concentration used, decreasing with
increasing concentration. However, dose-dependent differences were not apparent over the second year
of the trial, with all three concentrations achieving similar slowing of myopia progression. The net
increases in myopia over the 2-year trial period were -0.49, -0.38, and -0.30 D for the 0.01%, 0.1%, and
0.5% concentrations, respectively.
Two of three more recent RCTs involved relatively high concentrations of atropine, being 1% (n=126) and
0.5% (n= 132) in the studies by Yi et al. (2015) and Wang et al. (2017), respectively. Both reported
hyperopic shifts in the atropine-treated groups, presumably reflecting, at least in part, the enduring strong
cycloplegic action of this treatment, while continued progression in control groups over the same period of
time was observed (i.e., +0.3 vs. -0.9 D and +0.5 vs. -0.8 D). Three lower concentrations of atropine,
0.01%, 0.025%, and 0.05%, were tested in the most recent of these studies, by Yam et al. (2018) (n =
438), who reported a concentration-dependent reduction in myopia progression, with the highest
concentration approximately halving the rate of axial elongation, as compared with the placebo control.
All concentrations were well tolerated.
Although retrospective studies typically lack the same level of control of key study design variables as
RCTs, overall their results are consistent with those of the RCTs just described. Several retrospective,
cohort studies have tested higher, 0.5% to 1.0% concentrations of atropine, reporting treatment effects
ranging from 70% to 100%, In one of four studies involving lower concentrations of atropine, children
treated with 0.025% atropine over 22 months were reported to progress by an average of -0.28 D per
year, compared with -0.75 D in untreated children (a reduction of 63%). Similarly, Fang and colleagues
(2010), using the same 0.025% atropine concentration with "premyopic" children (spherical equivalent
refractive error: +1 to 1 D), reported a reduction in incident myopia and reduced progression compared
with controls (21% versus 54%, -0.14 vs. -0.58 D). Wu and colleagues also noted reduced progression
with atropine treatments, although interpretation of their study findings is complicated by the variation in
atropine concentrations used to treat individual patients over the 4.5-year monitoring period, between
0.05% and 0.1%; the overall mean progression was -0.23 D per year, compared with -0.86 D per year in
historical "controls." A surprisingly low average myopia progression of -0.1 D per year was reported for
0.01% atropine in the only retrospective study involving this concentration, referenced against a control
rate of -0.6 D per year, 112 although interestingly, this study included children of both Asian and
Caucasian ethnicity.
Timolol –
The RCT by Jensen 130 had three treatment arms: SV spectacles (n = 51), bifocal spectacles (n = 57),
and SV spectacles + timolol (n = 51). The timolol arm used 0.25% timolol maleate, twice a day. Children
were followed for 2 years, with additional examinations 1 year after completion of the trial. The results
were generally disappointing, with mean myopia progression over the 2-year study period in the control
and timolol groups being almost identical , and not significantly different from each other. This was despite
confirmation that timolol lowered IOP significantly, by approximately 3 mm Hg, with those with high IOP
showing the largest treatment effect. Also, although there appeared to be a trend toward increasing
noncompliance over time, progression rates did not appear to reflect compliance. Curiously, higher
progression rates appeared associated with higher IOP in the control group, with this relationship
reaching statistical significance for the girls, with a similar but not significant trend for boys.