Photoelectric effect, phenomenon in which electrically charged particles are released from or within a material when it absorbs electromagnetic radiation. The effect is often defined as the ejection of electrons from a metal plate when light falls on it. In a broader definition, the radiant energy may be infrared, visible, or ultraviolet light, X-rays, or gamma rays; the material may be a solid, liquid, or gas; and the released particles may be ions (electrically charged atoms or molecules) as well as electrons. The phenomenon was fundamentally significant in the development of modern physics because of the puzzling questions it raised about the nature of light—particle versus wavelike behaviour—that were finally resolved by Albert Einstein in 1905. The effect remains important for research in areas from materials science to astrophysics, as well as forming the basis for a variety of useful devices.
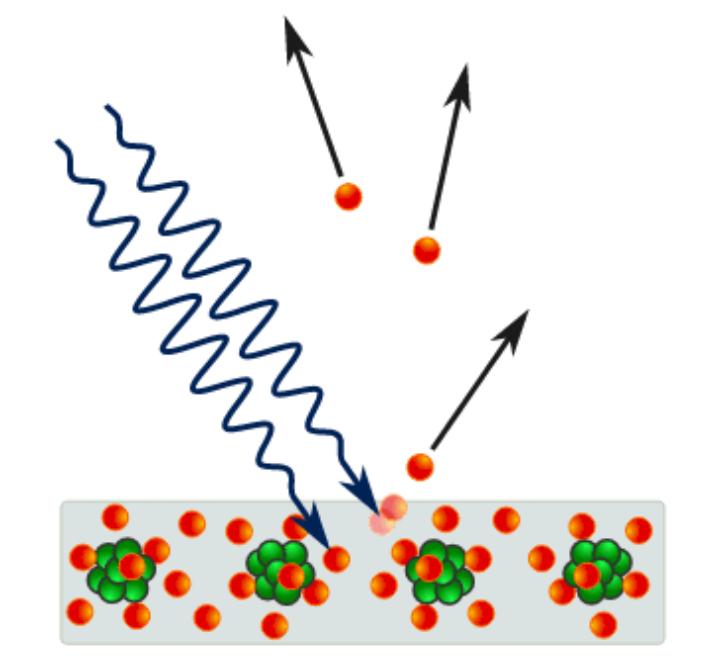
The experimental results disagree with classical electromagnetism, which predicts that continuous light waves transfer energy to electrons, which would then be emitted when they accumulate enough energy. An alteration in the intensity of light would theoretically change the kinetic energy of the emitted electrons, with sufficiently dim light resulting in a delayed emission. The experimental results instead show that electrons are dislodged only when the light exceeds a certain frequency—regardless of the light’s intensity or duration of exposure. Because a low-frequency beam at a high intensity could not build up the energy required to produce photoelectrons, as it would have if light’s energy were coming from a continuous wave, Albert Einstein proposed that a beam of light is not a wave propagating through space, but a swarm of discrete energy packets, known as photons.
Emission of conduction electrons from typical metals requires a few electron-volt (eV) light quanta, corresponding to short-wavelength visible or ultraviolet light. In extreme cases, emissions are induced with photons approaching zero energy, like in systems with negative electron affinity and the emission from excited states, or a few hundred keV photons for core electrons in elements with a high atomic number.
[1] Study of the photoelectric effect led to important steps in understanding the quantum nature of light and electrons and influenced the formation of the concept of wave–particle duality.
[2] Other phenomena where light affects the movement of electric charges include the photoconductive effect, the photovoltaic effect, and the photoelectrochemical effect.
The photoelectric effect represents an interaction between light and matter that cannot be explained by classical physics, which describes light as an electromagnetic wave. One inexplicable observation was that the maximum kinetic energy of the released electrons did not vary with the intensity of the light, as expected according to the wave theory, but was proportional instead to the frequency of the light. What the light intensity did determine was the number of electrons released from the metal (measured as an electric current). Another puzzling observation was that there was virtually no time lag between the arrival of radiation and the emission of electrons.
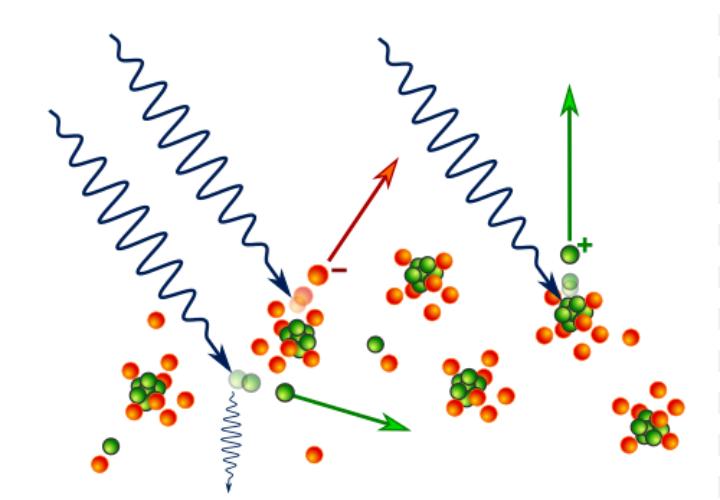
Photoelectric principles:
According to quantum mechanics, electrons bound to atoms occur in specific electronic configurations. The highest energy configuration (or energy band) that is normally occupied by electrons for a given material is known as the valence band, and the degree to which it is filled largely determines the material’s electrical conductivity. In a typical conductor (metal), the valence band is about half filled with electrons, which readily move from atom to atom, carrying a current. In a good insulator, such as glass or rubber, the valence band is filled, and these valence electrons have very little mobility. Like insulators, semiconductors generally have their valence bands filled, but, unlike insulators, very little energy is required to excite an electron from the valence band to the next allowed energy band—known as the conduction band, because any electron excited to this higher energy level is relatively free. For example, the “bandgap” for silicon is 1.12 eV (electron volts), and that of gallium arsenide is 1.42 eV. This is in the range of energy carried by photons of infrared and visible light, which can therefore raise electrons in semiconductors to the conduction band. (For comparison, an ordinary flashlight battery imparts 1.5 eV to each electron that passes through it. Much more energetic radiation is required to overcome the bandgap in insulators.) Depending on how the semiconducting material is configured, this radiation may enhance its electrical conductivity by adding to an electric current already induced by an applied voltage (see photoconductivity), or it may generate a voltage independently of any external voltage sources (see photovoltaic effect).
In the photovoltaic effect, a voltage is generated when the electrons freed by the incident light are separated from the holes that are generated, producing a difference in electrical potential. This is typically done by using a p-n junction rather than a pure semiconductor. A p-n junction occurs at the juncture between p-type (positive) and n-type (negative) semiconductors. These opposite regions are created by the addition of different impurities to produce excess electrons (n-type) or excess holes (p-type). Illumination frees electrons and holes on opposite sides of the junction to produce a voltage across the junction that can propel current, thereby converting light into electrical power.
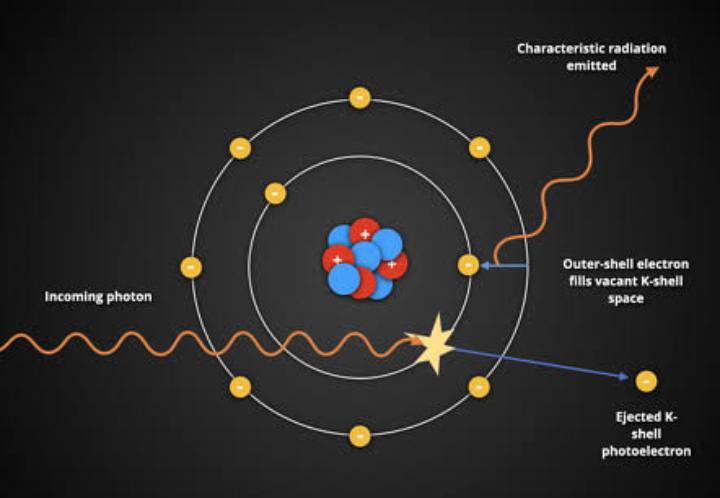
Other photoelectric effects are caused by radiation at higher frequencies, such as X-rays and gamma rays. These higher-energy photons can even release electrons near the atomic nucleus, where they are tightly bound. When such an inner electron is ejected, a higher-energy outer electron quickly drops down to fill the vacancy. The excess energy results in the emission of one or more additional electrons from the atom, which is called the Auger effect.
Also seen at high photon energies is the Compton effect, which arises when an X-ray or gamma-ray photon collides with an electron. The effect can be analyzed by the same principles that govern the collision between any two bodies, including conservation of momentum. The photon loses energy to the electron, a decrease that corresponds to an increased photon wavelength according to Einstein’s relation E = hc/λ. When the collision is such that the electron and the photon part at right angles to each other, the photon’s wavelength increases by a characteristic amount called the Compton wavelength, 2.43 × 10−12 metre.