Glaucoma, often termed the “silent thief of sight,”
remains one of the leading causes of irreversible
blindness worldwide. The early stages are
asymptomatic, making early detection a crucial
factor in preventing vision loss. In primary eye care
settings, screening for glaucoma has traditionally
relied heavily on two primary parameters: intraocular
pressure (IOP) and cup-to-disc ratio (CDR).
However, these measures, while useful, can miss
early glaucomatous changes and potentially delay
diagnosis.
This blog explores the limitations of relying solely on
IOP and CDR and highlights comprehensive
strategies and diagnostic advancements that
primary eye care providers can adopt to detect
glaucoma at its earliest stages.
Understanding the Basics: IOP and CDR
Intraocular Pressure (IOP)
IOP measurement using tonometry is a common first
step in glaucoma screening. Elevated IOP is a
known risk factor for glaucoma, particularly primary
open-angle glaucoma (POAG). However, not all
glaucoma patients present with high IOP. Studies
have shown that:
Normal-tension glaucoma (NTG) patients can
develop optic nerve damage even when IOP is
within the normal range (10–21 mmHg).
Conversely, ocular hypertension (OHT) patients may
never develop glaucoma despite consistently
elevated IOP.
This discrepancy illustrates that IOP alone lacks the
sensitivity and specificity needed for early detection.
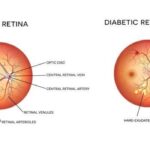
Cup-to-Disc Ratio (CDR)
The optic nerve head (ONH) evaluation, particularly
the vertical cup-to-disc ratio, has long been used to
detect glaucomatous changes. A higher CDR
(commonly >0.6) or asymmetry between eyes may
indicate optic nerve damage. However:
CDR is subjective and varies with the size of the
optic disc.
Physiological large cups may be misinterpreted as
glaucomatous.
Early focal changes in the neuroretinal rim may go
unnoticed.
Therefore, an over-reliance on CDR can result in
both false positives and false negatives.
The Need to Look Beyond: Why Primary Eye Care
Must Evolve
Missed Diagnoses in Early Stages
Early glaucoma is typically asymptomatic. By the
time a patient notices vision loss, significant retinal
ganglion cell (RGC) damage has occurred. Studies
estimate that up to 40% of RGCs may be lost before
standard visual field (VF) defects are detectable.
Relying only on IOP and CDR can lead to missed
cases, especially in patients with NTG or subtle optic
nerve changes.
Variability and Subjectivity
Both IOP and CDR assessments are influenced by
interobserver variability, instrument accuracy, and
patient cooperation. These subjective elements
reduce diagnostic reliability, particularly in primary
settings with high patient volumes and limited
specialist support.
A Comprehensive Approach to Early Detection
To improve early glaucoma detection, primary eye
care must adopt a more holistic and structured
approach involving the following:
1. Detailed Optic Nerve Head Evaluation :
Beyond measuring CDR, clinicians should:
Assess rim integrity using the ISNT Rule (Inferior >
Superior > Nasal > Temporal). Rim thinning in
inferior or superior areas is often an early
glaucomatous sign.
Look for localized notching, disc hemorrhages, and
parapapillary atrophy.
Compare both eyes for asymmetry, even when each
CDR appears “normal.”
Using slit-lamp biomicroscopy with a +78D or +90D
lens enhances optic nerve visualization and
improves detection accuracy.
2. Optical Coherence Tomography (OCT) :
OCT has revolutionized the detection of early
structural glaucomatous damage. It offers:
Objective, reproducible measurements of retinal
nerve fiber layer (RNFL) and ganglion cell complex
(GCC).
Ability to detect pre-perimetric glaucoma—structural
damage before visual field loss is evident.
In primary settings, non-mydriatic or portable OCT
devices can be valuable additions for early
screening, especially in high-risk patients.
3. Visual Field Testing (Perimetry) :
Standard automated perimetry (SAP) remains a
critical tool for glaucoma diagnosis. In early
detection:
24-2 or 10-2 test patterns may reveal early
functional defects.
Repeating tests is essential to confirm subtle
changes and reduce learning effects.
In resource-limited settings, frequency doubling
technology (FDT) perimetry can provide quicker,
cost-effective functional screening with good
sensitivity.
4. Corneal Thickness and Biomechanics :
Central corneal thickness (CCT) influences IOP
readings. Thin corneas can mask elevated IOP,
underestimating glaucoma risk. Conversely, thick
corneas may lead to overestimation.
CCT measurement (pachymetry) allows better risk
assessment and more accurate IOP interpretation.
Devices like the Ocular Response Analyzer (ORA)
also assess corneal hysteresis—another
independent risk factor for glaucoma progression.
5. Risk Factor Analysis :
A thorough patient history and risk evaluation should
guide screening intensity. Important risk factors
include:
Family history of glaucoma
Age >40, especially in African, Hispanic, or Asian
populations
Myopia (associated with POAG)
Systemic diseases like diabetes, hypertension, and
obstructive sleep apnea
Steroid use, especially topical or systemic
6. Digital Palpitation or Digital Tonometry :
In rural or resource-constrained primary eye
care settings, where standard tonometry tools
may not be available, digital palpation — also
known as digital tonometry — is still practiced by
trained eye care professionals. In this method,
the examiner uses their index fingers to gently
press the patient’s upper eyelid (over the globe)
to estimate the relative hardness of the eye.
When and How It Is Used:
Often used during field visits, emergency
settings, or when the patient is uncooperative.
The eye Is palpated through closed lids, and the
examiner compares the firmness with a known
normal.
While highly subjective, experienced
practitioners can detect significantly raised IOP,
which can prompt urgent referrals
Limitations:
Cannot provide exact IOP values.
Requires significant clinical experience.
Not reliable for borderline cases or subtle IOP
changes.
Stratifying patients by risk allows prioritization of
resources and follow-up strategies.
Enhancing Clinical Skills
Training optometrists and primary care providers in
detailed ONH evaluation, interpretation of OCT and
VF results, and risk profiling is vital. Skill-building
workshops and online certifications can improve
confidence and accuracy in glaucoma detection.
Adopting Technology
While advanced diagnostics like OCT may be
considered high-cost, shared resources, portable
devices, and screening camps using mobile
technology can bring these tools to underserved
areas.
Affordable technologies such as:
iCare tonometry (requiring no anesthesia or
calibration)
Smartphone-based fundus cameras
AI-integrated visual field apps
Collaborative Care Models
Establishing referral pathways with ophthalmologists
and glaucoma specialists ensures continuity of care.
Primary providers should maintain detailed records
and refer suspicious cases promptly for confirmatory
testing and management.
A hub-and-spoke model, with specialists guiding
peripheral clinics, allows early intervention and
better resource allocation.
Case Scenarios: Detecting Glaucoma Early
Case 1: The Silent Threat :
A 55-year-old woman presents for routine vision
testing. IOP is 16 mmHg OU; CDR is 0.4 in both
eyes. No symptoms.
Using OCT, RNFL thinning is observed in the
superior quadrant of the right eye. Visual field shows
early superior arcuate scotoma.
Diagnosis: Early normal-tension glaucoma. This
case would likely have been missed with IOP and
CDR alone.
Case 2: The Overlooked Risk :
A 45-year-old male with a family history of glaucoma
reports occasional blurred vision. IOP is 22 mmHg
OD and 24 mmHg OS. CDR 0.5 OU with no obvious
asymmetry.
Further testing reveals thin corneas (CCT 485 µm),
inferior rim thinning on ONH exam, and borderline
defects on FDT perimetry.
Diagnosis: Ocular hypertension with early structural
damage. Early treatment can prevent progression to
POAG.
Future Directions: The Role of AI and Telemedicine :
Artificial intelligence (AI) is increasingly being
integrated into glaucoma screening:
AI algorithms can analyze fundus images, OCT
scans, and visual fields with impressive accuracy.
Tele-glaucoma platforms allow rural or primary care
centers to upload data for expert review, enabling
mass screening and remote diagnosis.
While still developing, these tools promise to make
early glaucoma detection more efficient, consistent,
and accessible.
Conclusion: A New Era in Glaucoma Detection In the battle against glaucoma, early detection
remains the most effective tool to prevent
irreversible blindness. While IOP and CDR have
long served as initial indicators, their limitations are
now widely recognized. A modern, multifactorial
approach—incorporating advanced imaging, visual
field testing, corneal assessment, risk profiling, and
emerging technologies—is essential.
Primary eye care providers are at the frontline of this
battle. With the right training, tools, and collaborative
strategies, they can significantly impact public health
by catching glaucoma early—when intervention can
still preserve vision and quality of life.